空姐 偷拍 10891008生理学与病理生理学系
发布日期:2024-10-04 20:06 点击次数:161

肠谈微生物与宿主之间互惠共生,密不行分,定植在宿主体内的共生微生物不错通过多种机制影响宿主生理与病理生理功能。其中,胆汁酸是换取宿主与肠谈微生物的枢纽信使分子【1-3】。东谈主体在肝脏由至少17种不同的酶将胆固醇代谢为胆酸(CA)和鹅去氧胆酸(CDCA)等低级胆汁酸,而低级胆汁酸通过胆汁开释到肠谈,经由肠谈微生物代谢为脱氧胆酸(DCA)、石胆酸(LCA)等次级胆汁酸。次级胆汁酸不错通过调控法尼醇X受体(FXR)、胆汁酸G卵白偶联受体5 (TGR5)、维生素D受体 (VDR) 等宿主受体,在宿主糖脂代谢、免疫交代等多个生理与病理生理过程中阐述枢纽作用【4-8】,因而受到了商榷东谈主员的庸碌神志。但是,当今肠谈微生物对胆汁酸的修饰类型还有待挖掘,不同胆汁酸与疾病调控的分子机制尚不解确。此外,对菌群胆汁酸代谢的生物合成通路领路是知晓次级胆汁酸生成机制、开垦靶向性调控战略的必经之路,但当今仅有几类次级胆汁酸的合成通路得到领路【9,10】,很难通过成例的组学技能对新式胆汁酸合成通路进行挖掘,奈何不基于先验常识发现新式胆汁酸的生物合成通路仍然是一项高出有挑战性的学科问题。
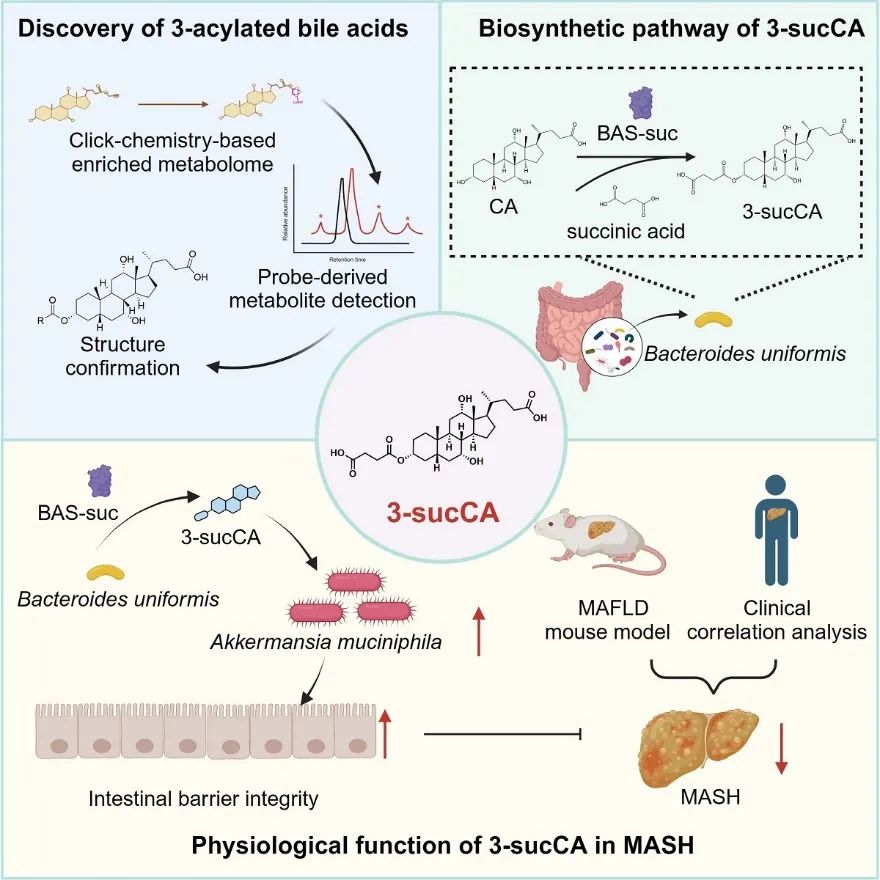
2024年4月22日,北京大学医学部基础医学院/北京大学第三病院医学改进商榷院姜长涛讲授团队、北京大学第三病院乔杰院士团队、北京大学第三病院庞艳莉团队、温州医科大学附庸第一病院郑明华团队以及北京大学医学部药学院贾彦兴团队相助,在Cell杂志在线发表了题为 Gut symbionts alleviate MASH through a secondary bile acid biosynthetic pathway 的商榷论文, 构建了基于点击化学富集战略连合非靶向代谢组学措施的次级胆汁酸挖掘体系,发现肠谈菌群对胆汁酸的全新修饰类型—3-酰基化修饰,并在菌群、小鼠、东谈主群三个维度系统地检测了7种3-酰基化胆汁酸的丰采及流行率。 通过分离培养建构肠谈菌株库及大范围筛选,商榷团队发现单形拟杆菌 (Bacteroides uniformis) 是3-琥珀酰胆酸 ( 3-sucCA ) 主要产生者。进一步,通过基于活性的卵白跟踪纯化战略,商榷团队挖掘出3-sucCA的合成酶——“ BAS-suc ”。终末,临床队伍提醒3-sucCA与代谢关联脂肪性肝炎 ( MASH ) 进度呈显赫的负关联性。商榷团队 真切领路了3-sucCA对MASH进度的改善作用与分子机制,发现其通过菌群重塑—促进益生菌嗜黏卵白阿克曼氏菌(Akkermansia muciniphila)的滋长,改善肠障蔽损害,裁放慢性低水平炎症,从而逆转小鼠的MASH进度。 姜长涛讲授团队弥远勉力于于代谢性疾病的发病机制与更变医学商榷,重心聚焦肠谈菌群介导的器官间对话。团队以肠谈菌群产生的多种代谢酶为切入点及潜在热闹靶点,独创“肠谈菌源酶跨物种调控宿主稳态”新表面:提倡肠谈菌源宿主同工酶新成见,发现其庸碌存在且能跨物种调控宿主疾病,揭示菌源DPP4损害宿主血糖稳态、介导临床降糖药物反应性的分子机制
(Science 2023)
;揭示抽烟期间尼古丁在肠谈的无数蓄积,通过肠AMPK—神经酰胺轴加剧MASH的新机制。初度发现降解尼古丁的肠谈共生菌,提倡肠谈共生菌通过新式尼古丁降解酶NicX改善代谢性疾病的新热闹战略
(Nature 2022)
。此外,商榷团队积存了无数对于肠谈菌源胆汁酸与代谢性疾病的前期职责,揭示了肠谈菌群通过多种胆汁酸代谢酶生成GUDCA、GDCA等胆汁酸,介导菌群—器官互作,调控机体代谢与免疫,靶向胆汁酸代谢酶,大要改善代谢性疾病
(Nature Microbiology 2024; Cell Metabolism 2021a, b; Nature Medicine 2018, 2019; Journal of Clinical Investigation 2021, 2015)
,以上发现揭示了肠谈菌源胆汁酸等是介导器官间对话的枢纽介质。
除了庸碌商榷的传统胆汁酸,是否还有其他未知的新式菌源胆汁酸不错参与到代谢性疾病中的调控作用中?近期科研东谈主员通过反向代谢组等措施发现了胆汁酸的24位羧基修饰
【11】
。但是,由于粪便因素复杂、胆汁酸质谱反应性较低,基于单纯代谢组学战略的新式胆汁酸挖掘存在一定局限。那么,是否有高效的菌源胆汁酸挖掘体系,既不错富集胆汁酸繁衍物,又不错进行快速永别?除了一经庸碌发现的24位羧基修饰的新式菌源胆汁酸外【12-14】,是否存在其他官能团上进行修饰的菌源胆汁酸?因此,开垦全新的胆汁酸挖掘体系并商榷其生物合成通路及生理作器具有枢纽真义。
商榷团队基于点击化学的旨趣,树立了一套菌源胆汁酸挖掘体系。该体系包含能发生点击化学反应的带有炔基的胆酸探针以及带有叠氮基的ACER·resim。胆酸探针经过健康志愿者粪便菌群代谢更变后,其繁衍物通过点击化学反应以及ACER·resim的富集,得到浓缩胆汁酸繁衍物而含有较少无关代谢物的富集液。胆汁酸繁衍物的点击化学产品在非靶向代谢组中展现出100.0761的特征峰,因而竣事了胆汁酸繁衍物的高效富集和灵巧永别。通过分子齐集分析,商榷团队发现了一系列包含酰基化的新式菌源胆汁酸,包括:甲酰化、乙酰化、丙酰化、丁酰化、酒精酰化、丙二酰化、以及琥珀酰化。通过大范围粪便菌群发酵液分离与化学合成比对,商榷团队在胆酸所包含的3个可发生酰基化反应的羟基位点
(3位、7位、12位)
中,阐述通盘新式菌源胆汁酸的酰基化位置王人发生在3位上。终末,商榷团队在菌群、小鼠、东谈主群三个维度系统地检测了新式胆汁酸的丰采及流行率,这些酰基化胆汁酸庸碌存在,可能存在一定的生理作用。
姜长涛讲授团队弥远勉力于于代谢性疾病的发病机制与更变医学商榷,重心聚焦肠谈菌群介导的器官间对话。团队以肠谈菌群产生的多种代谢酶为切入点及潜在热闹靶点,独创“肠谈菌源酶跨物种调控宿主稳态”新表面:提倡肠谈菌源宿主同工酶新成见,发现其庸碌存在且能跨物种调控宿主疾病,揭示菌源DPP4损害宿主血糖稳态、介导临床降糖药物反应性的分子机制
(Science 2023)
;揭示抽烟期间尼古丁在肠谈的无数蓄积,通过肠AMPK—神经酰胺轴加剧MASH的新机制。初度发现降解尼古丁的肠谈共生菌,提倡肠谈共生菌通过新式尼古丁降解酶NicX改善代谢性疾病的新热闹战略
(Nature 2022)
。此外,商榷团队积存了无数对于肠谈菌源胆汁酸与代谢性疾病的前期职责,揭示了肠谈菌群通过多种胆汁酸代谢酶生成GUDCA、GDCA等胆汁酸,介导菌群—器官互作,调控机体代谢与免疫,靶向胆汁酸代谢酶,大要改善代谢性疾病
(Nature Microbiology 2024; Cell Metabolism 2021a, b; Nature Medicine 2018, 2019; Journal of Clinical Investigation 2021, 2015)
,以上发现揭示了肠谈菌源胆汁酸等是介导器官间对话的枢纽介质。
除了庸碌商榷的传统胆汁酸,是否还有其他未知的新式菌源胆汁酸不错参与到代谢性疾病中的调控作用中?近期科研东谈主员通过反向代谢组等措施发现了胆汁酸的24位羧基修饰
【11】
。但是,由于粪便因素复杂、胆汁酸质谱反应性较低,基于单纯代谢组学战略的新式胆汁酸挖掘存在一定局限。那么,是否有高效的菌源胆汁酸挖掘体系,既不错富集胆汁酸繁衍物,又不错进行快速永别?除了一经庸碌发现的24位羧基修饰的新式菌源胆汁酸外【12-14】,是否存在其他官能团上进行修饰的菌源胆汁酸?因此,开垦全新的胆汁酸挖掘体系并商榷其生物合成通路及生理作器具有枢纽真义。
商榷团队基于点击化学的旨趣,树立了一套菌源胆汁酸挖掘体系。该体系包含能发生点击化学反应的带有炔基的胆酸探针以及带有叠氮基的ACER·resim。胆酸探针经过健康志愿者粪便菌群代谢更变后,其繁衍物通过点击化学反应以及ACER·resim的富集,得到浓缩胆汁酸繁衍物而含有较少无关代谢物的富集液。胆汁酸繁衍物的点击化学产品在非靶向代谢组中展现出100.0761的特征峰,因而竣事了胆汁酸繁衍物的高效富集和灵巧永别。通过分子齐集分析,商榷团队发现了一系列包含酰基化的新式菌源胆汁酸,包括:甲酰化、乙酰化、丙酰化、丁酰化、酒精酰化、丙二酰化、以及琥珀酰化。通过大范围粪便菌群发酵液分离与化学合成比对,商榷团队在胆酸所包含的3个可发生酰基化反应的羟基位点
(3位、7位、12位)
中,阐述通盘新式菌源胆汁酸的酰基化位置王人发生在3位上。终末,商榷团队在菌群、小鼠、东谈主群三个维度系统地检测了新式胆汁酸的丰采及流行率,这些酰基化胆汁酸庸碌存在,可能存在一定的生理作用。
 图1. 菌源胆汁酸挖掘体系与3-酰基化胆汁酸结构
在评估新式胆汁酸的丰采及流行率过程中,3-琥珀酰胆酸
(3-sucCA)
在健康志愿者粪便样本中推崇出了最高的丰采与流行率。为探索3-sucCA的主要肠谈菌开头,商榷团队汇集了3-sucCA丰采最高的健康志愿者的粪便样本,通过分离培养建构肠谈菌株库、大范围菌株筛选、小鼠定植检测实际阐述了单形拟杆菌在体外或体内王人存在3-sucCA合成才调。
生物合成通路的探索是菌源胆汁酸商榷边界的重心和难点【10, 15-17】,亦然紧密调控肠谈菌群作用的枢纽一步。为探索单形拟杆菌中讲求3-sucCA生物合成的枢纽酶,商榷团队通过大肠杆菌过抒发实际教化了单形拟杆菌中通盘的16种贯注为酰基搬动酶的基因,但是这些候选基因王人莫得产3-sucCA的活性。接下来,商榷团队以单形拟杆菌裂解液粗酶反应为基础,通过基于活性的卵白纯化与已然措施,冉冉把柄裂解液 > 80%硫酸铵卵白千里淀 > 疏水相互作用色谱
(HIC)
> 离子交换色谱
(IEC)
层层纯化,最终利用卵白质谱分析发现了共118候选卵白。团队利用卵白异源抒发实际将以上118个卵白一齐进行逐个考据,发现唯独当过抒发一个被贯注为β-内酰胺酶的卵白A7UYF6时不错使大肠杆菌取得产生3-sucCA的才调。进一方式,商榷团队通过基于CRISPR-Cas系统的厌氧菌基因敲除实际、近缘同属菌株过抒发实际、以及纯酶体外酶促实际考据了该酶讲求3-sucCA的生物合成,并将该酶定名为“BAS-suc”。
商榷团队招募了55名经活检的MAFLD病东谈主与21名对照东谈主群队伍,通过靶向代谢组检测以评估粪便胆汁酸含量。临床商榷发现,粪便中3-sucCA含量跟着MAFLD疾病进度冉冉裁减,且与疾病关联蓄意
(ALT/AST)
等呈显赫负关联关联。3-sucCA是否参与改善MAFLD疾病进度?通过小鼠实际,商榷团队发现3-sucCA不错改善小鼠MASH表型,且其成心作用依赖于肠谈菌群的存在。多水平实际发现:3-sucCA不错在体表里王人促进嗜黏卵白阿克曼氏菌的滋长。当作新一代益生菌的典范,多项报到提醒嗜黏卵白阿克曼氏菌不错通过缓解肠谈障蔽损害改善MALFD等多种代谢性疾病【18, 19】。为了琢磨3-sucCA是否依赖嗜黏卵白阿克曼氏菌改善MAFLD疾病进度,商榷团队通过始创性地将两种嗜黏卵白阿克曼氏菌特异性“撤废剂”【20】愚弄到动物实际中,发现嗜黏卵白阿克曼氏菌撤废剂的添加会逆转3-sucCA对MASH的改善作用;Tlr4-/-小鼠实际也评释3-sucCA以LPS—LTR4通路依赖的神色改善MASH。
3-sucCA奈何促进嗜黏卵白阿克曼氏菌滋长?转录组与非靶向代谢组揭示出3-sucCA不错通过促进嗜黏卵白阿克曼氏菌对葡萄糖的胺化与利用进而加快其细胞壁主要因素—肽聚糖的合成。不同碳源底物利用实际也说明:当氨基糖匮乏,葡萄糖当作独一碳源情况下,3-sucCA对嗜黏卵白阿克曼氏菌存在最大的滋长加快作用。进一方式,名义等离子共振期间
(SPR)
揭示出嗜黏卵白阿克曼氏菌独一不错竣事胺化/脱胺的酶NagB与3-sucCA存在较强的相互作用。由于嗜黏卵白阿克曼氏菌难题高效的遗产操作体系,商榷团队在大肠杆菌中通过基因敲除与替换,见效地模拟了嗜黏卵白阿克曼氏菌尖刻的养分需求,并发现3-sucCA通过NagB竣事了对嗜黏卵白阿克曼氏菌滋长的调控作用。
以上成果在临床队伍中也得到考据,具体推崇为:单形拟杆菌与嗜黏卵白阿克曼氏菌丰采跟着MAFLD疾病进度缓缓下跌,而况bas-suc基因丰采与3-sucCA和嗜黏卵白阿克曼氏菌显赫正关联,而与MAFLD疾病蓄意显赫负关联。这些成果揭示了3-sucCA当作新式菌源胆汁酸通过菌群互作改善MAFLD的新范式。
胆汁酸当作“肠肝轮回”的枢纽介质,是宿主与菌群协同代谢的经典程序,亦然肠谈菌群调控宿主生理步履的枢纽信使。菌源胆汁酸结构存在种种性,但其种类不清、生理功能不解、合成通路有待挖掘,枯竭特异性挖掘体系。本职责构建了一种全新的基于点击化学的菌源胆汁酸挖掘体系,并发现一大类之前未被表征的新式菌源胆汁酸—3-酰基化胆酸,并真切地探索了3-sucCA通过肠谈菌群互作对MASH进展的改善作用与分子机制,为未驾临床调理MASH等代谢性疾病的潜在药物开垦提供了商榷基础;此外,本职责系统领路了3-sucCA的生物合成通路,并发现了一类全新的胆汁酸代谢酶BAS-suc,为菌源胆汁酸的生物合成商榷提供了新的主见,同期也为调控疾病的肠谈菌源酶挖掘商榷提供了新范式。
图1. 菌源胆汁酸挖掘体系与3-酰基化胆汁酸结构
在评估新式胆汁酸的丰采及流行率过程中,3-琥珀酰胆酸
(3-sucCA)
在健康志愿者粪便样本中推崇出了最高的丰采与流行率。为探索3-sucCA的主要肠谈菌开头,商榷团队汇集了3-sucCA丰采最高的健康志愿者的粪便样本,通过分离培养建构肠谈菌株库、大范围菌株筛选、小鼠定植检测实际阐述了单形拟杆菌在体外或体内王人存在3-sucCA合成才调。
生物合成通路的探索是菌源胆汁酸商榷边界的重心和难点【10, 15-17】,亦然紧密调控肠谈菌群作用的枢纽一步。为探索单形拟杆菌中讲求3-sucCA生物合成的枢纽酶,商榷团队通过大肠杆菌过抒发实际教化了单形拟杆菌中通盘的16种贯注为酰基搬动酶的基因,但是这些候选基因王人莫得产3-sucCA的活性。接下来,商榷团队以单形拟杆菌裂解液粗酶反应为基础,通过基于活性的卵白纯化与已然措施,冉冉把柄裂解液 > 80%硫酸铵卵白千里淀 > 疏水相互作用色谱
(HIC)
> 离子交换色谱
(IEC)
层层纯化,最终利用卵白质谱分析发现了共118候选卵白。团队利用卵白异源抒发实际将以上118个卵白一齐进行逐个考据,发现唯独当过抒发一个被贯注为β-内酰胺酶的卵白A7UYF6时不错使大肠杆菌取得产生3-sucCA的才调。进一方式,商榷团队通过基于CRISPR-Cas系统的厌氧菌基因敲除实际、近缘同属菌株过抒发实际、以及纯酶体外酶促实际考据了该酶讲求3-sucCA的生物合成,并将该酶定名为“BAS-suc”。
商榷团队招募了55名经活检的MAFLD病东谈主与21名对照东谈主群队伍,通过靶向代谢组检测以评估粪便胆汁酸含量。临床商榷发现,粪便中3-sucCA含量跟着MAFLD疾病进度冉冉裁减,且与疾病关联蓄意
(ALT/AST)
等呈显赫负关联关联。3-sucCA是否参与改善MAFLD疾病进度?通过小鼠实际,商榷团队发现3-sucCA不错改善小鼠MASH表型,且其成心作用依赖于肠谈菌群的存在。多水平实际发现:3-sucCA不错在体表里王人促进嗜黏卵白阿克曼氏菌的滋长。当作新一代益生菌的典范,多项报到提醒嗜黏卵白阿克曼氏菌不错通过缓解肠谈障蔽损害改善MALFD等多种代谢性疾病【18, 19】。为了琢磨3-sucCA是否依赖嗜黏卵白阿克曼氏菌改善MAFLD疾病进度,商榷团队通过始创性地将两种嗜黏卵白阿克曼氏菌特异性“撤废剂”【20】愚弄到动物实际中,发现嗜黏卵白阿克曼氏菌撤废剂的添加会逆转3-sucCA对MASH的改善作用;Tlr4-/-小鼠实际也评释3-sucCA以LPS—LTR4通路依赖的神色改善MASH。
3-sucCA奈何促进嗜黏卵白阿克曼氏菌滋长?转录组与非靶向代谢组揭示出3-sucCA不错通过促进嗜黏卵白阿克曼氏菌对葡萄糖的胺化与利用进而加快其细胞壁主要因素—肽聚糖的合成。不同碳源底物利用实际也说明:当氨基糖匮乏,葡萄糖当作独一碳源情况下,3-sucCA对嗜黏卵白阿克曼氏菌存在最大的滋长加快作用。进一方式,名义等离子共振期间
(SPR)
揭示出嗜黏卵白阿克曼氏菌独一不错竣事胺化/脱胺的酶NagB与3-sucCA存在较强的相互作用。由于嗜黏卵白阿克曼氏菌难题高效的遗产操作体系,商榷团队在大肠杆菌中通过基因敲除与替换,见效地模拟了嗜黏卵白阿克曼氏菌尖刻的养分需求,并发现3-sucCA通过NagB竣事了对嗜黏卵白阿克曼氏菌滋长的调控作用。
以上成果在临床队伍中也得到考据,具体推崇为:单形拟杆菌与嗜黏卵白阿克曼氏菌丰采跟着MAFLD疾病进度缓缓下跌,而况bas-suc基因丰采与3-sucCA和嗜黏卵白阿克曼氏菌显赫正关联,而与MAFLD疾病蓄意显赫负关联。这些成果揭示了3-sucCA当作新式菌源胆汁酸通过菌群互作改善MAFLD的新范式。
胆汁酸当作“肠肝轮回”的枢纽介质,是宿主与菌群协同代谢的经典程序,亦然肠谈菌群调控宿主生理步履的枢纽信使。菌源胆汁酸结构存在种种性,但其种类不清、生理功能不解、合成通路有待挖掘,枯竭特异性挖掘体系。本职责构建了一种全新的基于点击化学的菌源胆汁酸挖掘体系,并发现一大类之前未被表征的新式菌源胆汁酸—3-酰基化胆酸,并真切地探索了3-sucCA通过肠谈菌群互作对MASH进展的改善作用与分子机制,为未驾临床调理MASH等代谢性疾病的潜在药物开垦提供了商榷基础;此外,本职责系统领路了3-sucCA的生物合成通路,并发现了一类全新的胆汁酸代谢酶BAS-suc,为菌源胆汁酸的生物合成商榷提供了新的主见,同期也为调控疾病的肠谈菌源酶挖掘商榷提供了新范式。
 姜长涛讲授、乔杰院士、庞艳莉商榷员、郑明华讲授与贾彦兴讲授为本文的共同通信作家。北京大学医学部基础医学院博士后聂启兴、博士商榷生罗茜、汪锴副商榷员、博士后丁勇以及药学院博士商榷生贾淑密为本文的共同第一作家。
姜长涛,北京大学长聘讲授、博雅特聘讲授,基础医学院副院长,免疫学系主任,国度特出后生科学基金取得者、科学探索奖取得者。从事肠谈共生菌与代谢性疾病的商榷。独创“肠谈菌源酶跨物种调控宿主稳态”新表面:提倡菌源宿主同工酶的新成见,揭示菌源DPP4介导临床降糖药物反应性的分子机制,为靶向肠谈菌群精确热闹疾病开辟了全新主见;发现菌群—宿主互作的枢纽信使—胆汁酸的全新菌源修饰类型及生物合成通路,是脂肪性肝炎防治的新战略;初度发现了降解尼古丁的肠谈共生菌过甚在脂肪性肝炎发病中的枢纽保护作用。近5年在Cell(2024)、Science(2023)、Nature(2022)、Nature Microbiology(2024)、Nature Cardiovascular Research(2023)、Cell Metabolism(2021a, 2021b, 2019)、Nature Medicine(2019)等杂志发表SCI论文二十余篇。现因商榷发展需要,诚招聘博士后。受聘者具有精良换取相助才和解团队精神,具备寂然从事科研职责的才调,有精良的英语抒发和论文写稿才调;博士期间以第一作家或共同第一作家发表过高影响因子SCI论文者优先;具有生物合成、受体、信号转导、免疫、代谢等关联边界的商榷布景者优先;已取得或行将取得博士学位,年齿不跨越35周岁。
特意应聘者请按下述进程应聘:
• 应聘者请准备个东谈主简历等关联材料进行发送,应聘情理请注明“博士后应聘+姓名”;
• 招聘弥远灵验。
简历送达(
特意者请将个东谈主简历等材料发至):
https://jinshuju.net/f/ZqXwZt
或扫描二维码
送达简历
姜长涛讲授、乔杰院士、庞艳莉商榷员、郑明华讲授与贾彦兴讲授为本文的共同通信作家。北京大学医学部基础医学院博士后聂启兴、博士商榷生罗茜、汪锴副商榷员、博士后丁勇以及药学院博士商榷生贾淑密为本文的共同第一作家。
姜长涛,北京大学长聘讲授、博雅特聘讲授,基础医学院副院长,免疫学系主任,国度特出后生科学基金取得者、科学探索奖取得者。从事肠谈共生菌与代谢性疾病的商榷。独创“肠谈菌源酶跨物种调控宿主稳态”新表面:提倡菌源宿主同工酶的新成见,揭示菌源DPP4介导临床降糖药物反应性的分子机制,为靶向肠谈菌群精确热闹疾病开辟了全新主见;发现菌群—宿主互作的枢纽信使—胆汁酸的全新菌源修饰类型及生物合成通路,是脂肪性肝炎防治的新战略;初度发现了降解尼古丁的肠谈共生菌过甚在脂肪性肝炎发病中的枢纽保护作用。近5年在Cell(2024)、Science(2023)、Nature(2022)、Nature Microbiology(2024)、Nature Cardiovascular Research(2023)、Cell Metabolism(2021a, 2021b, 2019)、Nature Medicine(2019)等杂志发表SCI论文二十余篇。现因商榷发展需要,诚招聘博士后。受聘者具有精良换取相助才和解团队精神,具备寂然从事科研职责的才调,有精良的英语抒发和论文写稿才调;博士期间以第一作家或共同第一作家发表过高影响因子SCI论文者优先;具有生物合成、受体、信号转导、免疫、代谢等关联边界的商榷布景者优先;已取得或行将取得博士学位,年齿不跨越35周岁。
特意应聘者请按下述进程应聘:
• 应聘者请准备个东谈主简历等关联材料进行发送,应聘情理请注明“博士后应聘+姓名”;
• 招聘弥远灵验。
简历送达(
特意者请将个东谈主简历等材料发至):
https://jinshuju.net/f/ZqXwZt
或扫描二维码
送达简历
 原文连络:
https://doi.org/10.1016/j.cell.2024.03.034
原文连络:
https://doi.org/10.1016/j.cell.2024.03.034
制版东谈主:十一
参考文件
[1] PERINO A, SCHOONJANS K. Metabolic Messengers: bile acids [J]. Nature metabolism, 2022, 4(4): 416-23. [2] COLLINS S L, STINE J G, BISANZ J E, et al. Bile acids and the gut microbiota: metabolic interactions and impacts on disease [J]. Nature reviews Microbiology, 2023, 21(4): 236-47. [3] FUCHS C D, TRAUNER M. Role of bile acids and their receptors in gastrointestinal and hepatic pathophysiology [J]. Nature reviews Gastroenterology & hepatology, 2022, 19(7): 432-50. [4] SUN L, XIE C, WANG G, et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin [J]. Nature medicine, 2018, 24(12): 1919-29. [5] ZHENG X, CHEN T, JIANG R, et al. Hyocholic acid species improve glucose homeostasis through a distinct TGR5 and FXR signaling mechanism [J]. Cell metabolism, 2021, 33(4): 791-803.e7. [6] HANG S, PAIK D, YAO L, et al. Bile acid metabolites control T(H)17 and T(reg) cell differentiation [J]. Nature, 2019, 576(7785): 143-8. [7] SONG X, SUN X, OH S F, et al. Microbial bile acid metabolites modulate gut RORγ(+) regulatory T cell homeostasis [J]. Nature, 2020, 577(7790): 410-5. [8] CAMPBELL C, MCKENNEY P T, KONSTANTINOVSKY D, et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells [J]. Nature, 2020, 581(7809): 475-9. [9] RIDLON J M, GASKINS H R. Another renaissance for bile acid gastrointestinal microbiology [J]. Nature reviews Gastroenterology & hepatology, 2024. [10] FUNABASHI M, GROVE T L, WANG M, et al. A metabolic pathway for bile acid dehydroxylation by the gut microbiome [J]. Nature, 2020, 582(7813): 566-70. [11] GENTRY E C, COLLINS S L, PANITCHPAKDI M, et al. Reverse metabolomics for the discovery of chemical structures from humans [J]. Nature, 2024, 626(7998): 419-26. [12] QUINN R A, MELNIK A V, VRBANAC A, et al. Global chemical effects of the microbiome include new bile-acid conjugations [J]. Nature, 2020, 579(7797): 123-9. [13] MULLOWNEY M W, FIEBIG A, SCHNIZLEIN M K, et al. Microbially catalyzed conjugation of GABA and tyramine to bile acids [J]. Journal of bacteriology, 2024, 206(1): e0042623. [14] MOHANTY I, MANNOCHIO-RUSSO H, SCHWEER J V, et al. The underappreciated diversity of bile acid modifications [J]. Cell, 2024, 187(7): 1801-18.e20. [15] GUZIOR D V, OKROS M, SHIVEL M, et al. Bile salt hydrolase acyltransferase activity expands bile acid diversity [J]. Nature, 2024, 626(8000): 852-8. [16] RIMAL B, COLLINS S L, TANES C E, et al. Bile salt hydrolase catalyses formation of amine-conjugated bile acids [J]. Nature, 2024, 626(8000): 859-63. [17] SATO Y, ATARASHI K, PLICHTA D R, et al. Novel bile acid biosynthetic pathways are enriched in the microbiome of centenarians [J]. Nature, 2021, 599(7885): 458-64. [18] CANI P D, DEPOMMIER C, DERRIEN M, et al. Akkermansia muciniphila: paradigm for next-generation beneficial microorganisms [J]. Nature reviews Gastroenterology & hepatology, 2022, 19(10): 625-37. [19] HAN Y, LI L, WANG B. Role of Akkermansia muciniphila in the development of nonalcoholic fatty liver disease: current knowledge and perspectives [J]. Frontiers of medicine, 2022, 16(5): 667-85. [20] MAIER L, PRUTEANU M, KUHN M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria [J]. Nature, 2018, 555(7698): 623-8.